Improving the molecular to digital interface for <fast, scalable, accessible, secure> molecular information systems
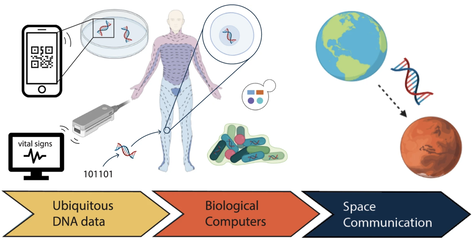
Integration of silicon and biomolecular systems is a promising direction of research that is motivated by potentially transformative outcomes, including new ways to build hybrid computer systems that integrate molecular data storage and processing with electronics, automated synthetic biology design-build-test- learn processes; and novel substrates for generalizable integration of electronics and life. While biomolecular computers cannot achieve the fast time scales at which semiconductor-based devices operate, biological parts have unique advantages. For example, they can be vastly smaller in scale, energetically efficient, and massively parallel. They can also more easily interface with natural biological systems for “near-data” processing. These features potentially enable wetware devices to perform in environments that traditional hardware cannot (e.g. inside the body), and even achieve sufficient computational throughput for otherwise expensive data processing tasks. Hence, the fast progress happening in DNA data storage, hybrid silicon-biomolecular sensors, and synthetic biology. However, continued progress in these areas calls for 1) more scalable mechanisms of accessing and operating on molecular data; 2) more efficient means of communication between electronic and molecular components; and 3) the ability to better understand how to reliably design and build biological components and systems at scale.
Integration of silicon and biomolecular systems is a promising direction of research that is motivated by potentially transformative outcomes, including new ways to build hybrid computer systems that integrate molecular data storage and processing with electronics, automated synthetic biology design-build-test- learn processes; and novel substrates for generalizable integration of electronics and life. While biomolecular computers cannot achieve the fast time scales at which semiconductor-based devices operate, biological parts have unique advantages. For example, they can be vastly smaller in scale, energetically efficient, and massively parallel. They can also more easily interface with natural biological systems for “near-data” processing. These features potentially enable wetware devices to perform in environments that traditional hardware cannot (e.g. inside the body), and even achieve sufficient computational throughput for otherwise expensive data processing tasks. Hence, the fast progress happening in DNA data storage, hybrid silicon-biomolecular sensors, and synthetic biology. However, continued progress in these areas calls for 1) more scalable mechanisms of accessing and operating on molecular data; 2) more efficient means of communication between electronic and molecular components; and 3) the ability to better understand how to reliably design and build biological components and systems at scale.